Crooks fluctuation theorem
The Crooks equation (CE)[1] is an equation in statistical mechanics that relates the work done on a system during a non-equilibrium transformation to the free energy difference between the final and the initial state of the transformation. During the non equilibrium transformation the system is at constant volume and in contact with a heat reservoir. The CE is named after the chemist Gavin E. Crooks (then at University of California) who discovered it in 1998. The CE is a special case of the more general fluctuation theorem.[2]
If we define a generic reaction coordinate of the system as a function of the Cartesian coordinates of the constituent particles ( e.g. , a distance between two particles), we can characterize every point along the reaction coordinate path by a parameter 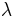 , such that
, such that 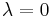 and
and 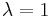 correspond to two ensembles of microstates (see microstate (statistical mechanics)) for which the reaction coordinate is constrained to different values. A dynamical process where
correspond to two ensembles of microstates (see microstate (statistical mechanics)) for which the reaction coordinate is constrained to different values. A dynamical process where 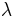 is externally driven from zero to one, according to an arbitrary time scheduling, will be referred as forward transformation , while the time reversal path will be indicated as backward transformation. Given these definitions, the CE sets a relation between the following four quantities:
is externally driven from zero to one, according to an arbitrary time scheduling, will be referred as forward transformation , while the time reversal path will be indicated as backward transformation. Given these definitions, the CE sets a relation between the following four quantities:
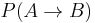 , i.e. the joint probability of taking a microstate
, i.e. the joint probability of taking a microstate 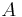 from the canonical ensemble corresponding to
from the canonical ensemble corresponding to 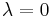 and of performing the forward transformation to the microstate B corresponding to
and of performing the forward transformation to the microstate B corresponding to 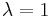 ;
;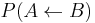 , i.e. the joint probability of taking the microstate
, i.e. the joint probability of taking the microstate 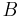 from the canonical ensemble corresponding to
from the canonical ensemble corresponding to 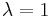 and of performing the backward transformation to the microstate
and of performing the backward transformation to the microstate 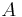 corresponding to
corresponding to 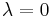 ;
;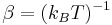 , where
, where 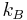 is the Boltzmann constant and
is the Boltzmann constant and 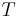 the temperature of the reservoir;
the temperature of the reservoir;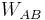 , i.e. the work done on the system during the forward transformation (from
, i.e. the work done on the system during the forward transformation (from 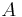 to
to 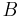 );
);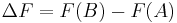 , i.e. the Helmholtz free energy difference between the state
, i.e. the Helmholtz free energy difference between the state 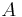 and
and 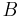 , represented by the canonical distribution of microstates having
, represented by the canonical distribution of microstates having 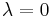 and
and 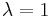 , respectively).
, respectively).
The CE equation reads as follows:
![\frac{P(A \rightarrow B)}
{P( A \leftarrow B)} = \exp [ \beta ( W_{A \rightarrow B} - \Delta F
)].](/2012-wikipedia_en_all_nopic_01_2012/I/35e1f034b0f1811758263a0ab4941c8e.png)
In the previous equation the difference 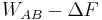 corresponds to the work dissipated in the forward transformation,
corresponds to the work dissipated in the forward transformation, 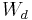 . The probabilities
. The probabilities 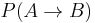 and
and 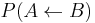 become identical when the transformation is performed at infinitely slow speed, i.e. for equilibrium transformations. In such case
become identical when the transformation is performed at infinitely slow speed, i.e. for equilibrium transformations. In such case 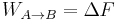 and
and 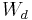 = 0.
= 0.
Using the time reversal relation 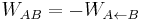 , and grouping together all the trajectories yielding the same work (in the forward and backward transformation), we can write the above equation in terms of the work distribution functions as follows
, and grouping together all the trajectories yielding the same work (in the forward and backward transformation), we can write the above equation in terms of the work distribution functions as follows
![P_{A \rightarrow B} (W) = P_{A
\leftarrow B}(- W) ~ \exp[\beta (W - \Delta F)].](/2012-wikipedia_en_all_nopic_01_2012/I/d50ad47e9b7420667a1d56553f490f14.png)
Note that for the backward transformation, the work distribution function must be evaluated by taking the work with the opposite sign. The two work distributions for the forward and backward processes cross at 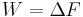 . This fact has been experimentally verified using optical tweezers for the process of unfolding and refolding of a small RNA hairpin and an RNA three-helix junction [1]
. This fact has been experimentally verified using optical tweezers for the process of unfolding and refolding of a small RNA hairpin and an RNA three-helix junction [1]
The CE implies the Jarzynski equality.
Notes
- ^ G. Crooks, "Entropy production fluctuation theorem and the nonequilibrium work relation for free energy differences", Physical Review E, 60, 2721 (1999)
- ^ Denis J. Evans & Debra J. Searles, "Equilibrium microstates which generate second law violating steady states", Physical Review, E 50, 1645 (1994)